Introduction Three TKIs, imatinib (IM), dasatinib (DAS) and nilotinib (NIL), are approved for frontline therapy in Italy. Choice of frontline TKI is based mainly on evaluation of patient's characteristics and clinical expectations. To avoid long term adverse events or potential drug interactions, elderly patients may start CML treatment with a frontline reduced dose of TKI (RD-TKI).
Aim To analyse outcome of CP-CML patients aged over 65 years in a large and unselected cohort treated with RD-TKI.
Methods We retrospectively evaluated 747 patients from 1/2012 to 12/2019 at 36 Hematology Centres participating at the “Campus CML” project.
Results Clinical features for the whole cohort according to frontline TKI initial dose are reported in Table 1. Among all patients, 605 (81%) were treated with standard dose (SD) while the remaining 142 (19%) with reduced dose (RD). As to frontline TKI, 579 patients (77%) received IM and 158 (23%) a 2G-TKI (DAS n=78, 49%; NIL n=80, 51%). Of the 142 RD-TKI, 122 (85.9%) started with IM, 14 (9.9%) with DAS and 6 (4.2%) with NIL. Median RD was 100 mg for IM (range 100-300), 20 mg for DAS (range 20-50) and 250 mg for NIL (range 150-300).
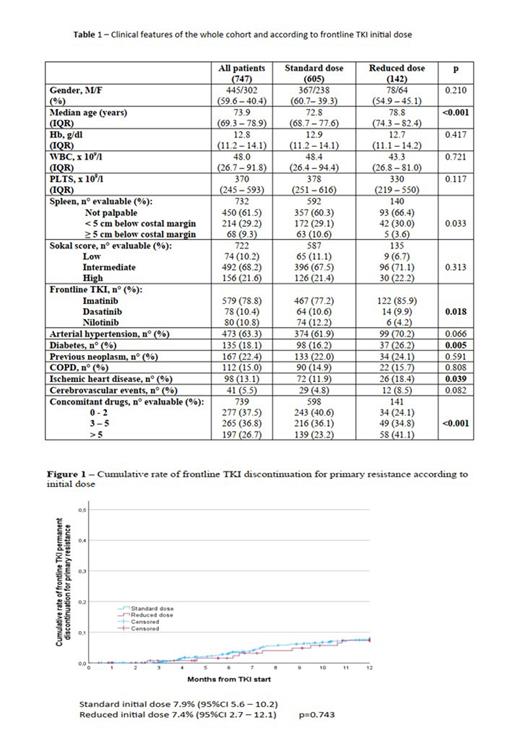
RD-TKI was mainly reported in IM treated patients (p=0.018), in elderly (p<0.001) and in patients with comorbidities, in particular diabetes (p=0.005) and ischemic heart disease (p=0.039). Number of concomitant drugs was also significantly associated with RD-TKI (p<0.001) probably to avoid drug interactions and subsequent toxicity. In detail, among RD-TKI, 41.1% of patients was treated with more than five concomitant drugs. Sokal score did not impact on TKI starting dose. No differences emerged between SD-TKI and RD-TKI in terms of haematological or extra-haematological toxicity. TKI frontline dose was not associated with difference in resistance, nor primary neither secondary resistance. Progression to blastic phase was reported in 1.2% of the whole population, none of which in RD-TKI. At 12 months no differences were noted in terms of achievement of major molecular response (MMR), obtained in 22.4% of SD-TKI treated patients and in 19% of RD-TKI treated patients. RD-TKI had inferior probability of deep molecular response (DMR) achievement (p=0.003), reported in 12.6% of patients. No differences were reported in 12-months cumulative rate of permanent discontinuation for any cause and for primary resistance between SD-TKI and RD-TKI as reported in Figure 1.
Conclusions RD-TKI was a frontline treatment strategy used mainly in frail elderly patients, with more comorbidities and concomitant therapies. RD-TKI did not impact on primary resistance leading to TKI switch. While no differences were reported in the rate of MMR, the rate of 12-months DMR achievement was inferior in RD-TKI, but this result need to be confirmed with longer follow-up.
Disclosures
Bucelli:Novartis/Incyte: Honoraria. Cavazzini:Novartis: Honoraria; Incyte: Honoraria; Pfizer: Honoraria. Bonifacio:Clinigen: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Elena:Blueprint Medicines Corporation, Cogent and Gilead: Other: Advisory Board Fees. Sportoletti:Abbvie, Janssen, Beigene, Astra Zeneca, Takeda, Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Stagno:Incyte, Novartis, Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Galimberti:Abbvie, Janssen, Novartis, Roche, Jazz, Astra Zeneca, Pfizer, Incyte: Speakers Bureau. Abruzzese:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy; Takeda: Consultancy; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Breccia:Novartis: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; BMS: Honoraria; AOP: Honoraria; AbbVie: Honoraria. Iurlo:Novartis, Pfizer, Incyte, BMS, GSK, AOP Health: Honoraria. Latagliata:BMS: Honoraria; Celgene: Honoraria; Janssen: Honoraria; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal